The Challenge
To quickly develop and make available an online service to enable business, other food and animal feed industry users and the public to search for and explore some details about CBD food products that are going through the authorisation process.
Background
Cannabidiol (CBD) food products are a particular type of food product, specifically a kind of ‘novel food’, i.e. a food that doesn’t have a ‘history of consumption’.
Regulated products are foods or materials involved in food production, or animal feed products that require authorisation before they can be sold in Great Britain. Those include, for example, extraction solvents, feed additives, flavourings, food additives, irradiated food and novel foods. In total there are 12 types of regulated products.
Information about regulated food and feed products that have been authorised for sale in Great Britain are provided in legislation and details of a range of types of them are available via one of our other services provided for FSA, The Regulated Food Products Register.
CBD products were newly classified as a regulated ‘novel food’ in Jan 2019. As part of the process to manage the authorisation of CBD products that were already for sale in Great Britain, the FSA set a deadline of 31 March 2021 to submit applications for authorisation. From the deadline date all new CBD products would require authorisation before going on sale, as do other regulated foods and animal feed.
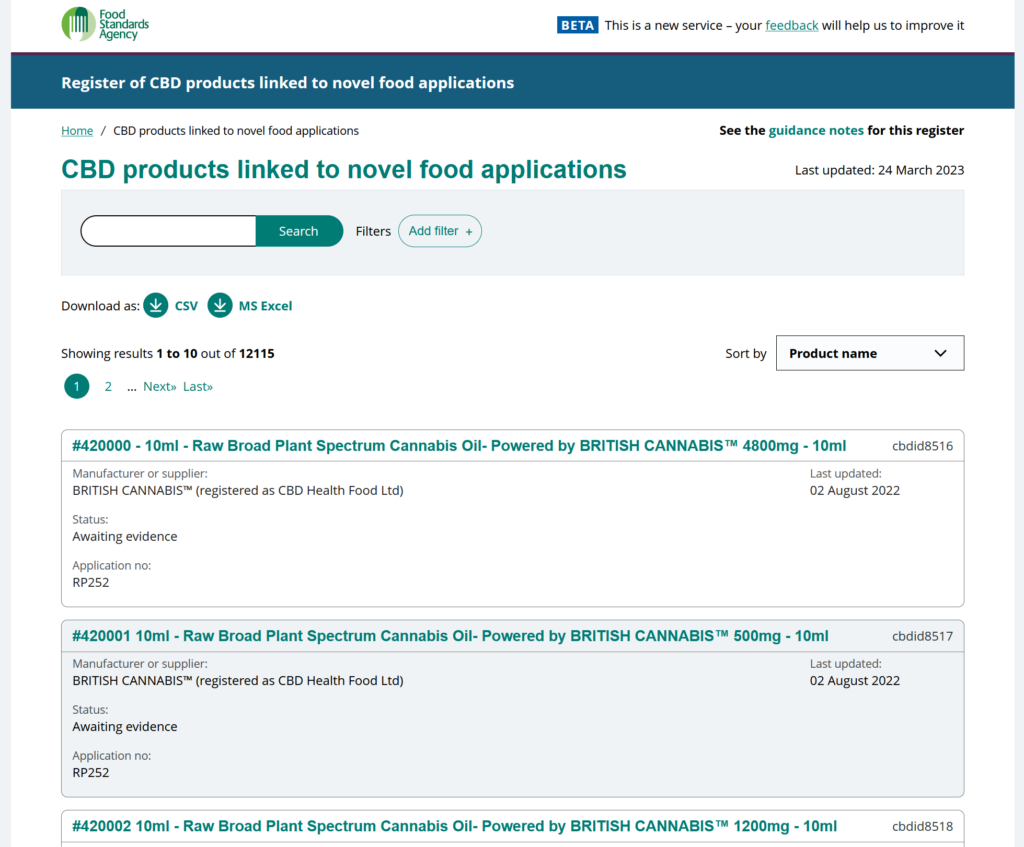
While the applications for existing products were being assessed, the FSA wished to enable the food industry and the public to be able to find out if a particular product (e.g. CBD based ingredient) had been registered for authorisation appropriately. This led to the relatively urgent need for an online ‘Register of CBD products linked to novel food applications’.
The register would also be updated to give the status of products as they have been assessed, i.e. Awaiting Evidence, Validated or Removed. All products marked ‘Removed’ must be withdrawn from the market.
Process
Building on our previous work on the Regulated Food Products Register, we worked with the relevant FSA teams to develop a bespoke register for the existing CBD products that were linked to the novel food applications.
This involved working to quickly understand the requirements of FSA, other stakeholders and end users, in order to develop a searchable register to enable them easily identify if a particular product was linked directly to an application under the regulations, and what the status of that application was.
To those ends, the register developed provides a simple means for FSA to keep the data updated and an easy to use, searchable interface for end users, enabling filtering of results by status and the ability to download results in standard CSV and Excel spreadsheet formats.

